NEGLAZYME is supplied as 5ml single use vials. Examine each vial before using. The solution should be clear to slightly opalescent and colorless to pale yellow. Do not use if the solution is discolored.
03:12
The recommended dose of NAGLAZYME® (galsulfase) is 1 mg/kg of body weight administered once weekly as an IV infusion over no less than 4 hours. Calculate the patient’s NAGLAZYME dose as follows:
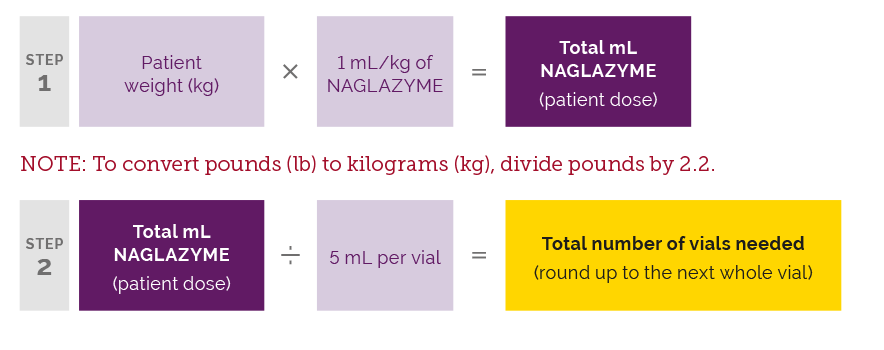
Before withdrawing NAGLAZYME from the vial, visually inspect each vial for particulate matter and discoloration.
The solution should be clear to slightly opalescent and colorless to pale yellow. Some translucency may be present in the solution. Do not use if the solution is discolored or if there is particulate matter in the solution. Do not freeze or shake vial.1
All MPS VI study patients, including those with weights as low as 14 kg, were infused using 250-mL total volume.
Consider using a 100‐mL infusion bag for patients who are1:
For a 250‐mL infusion bag:
For a 100‐mL infusion bag:
Using an 18‐gauge non‐filter needle, slowly withdraw the calculated dose of NAGLAZYME from the appropriate number of vials, using caution to avoid excessive agitation, bubbles, and foaming.
Slowly add the NAGLAZYME to the 0.9% Sodium Chloride Injection, USP, bag, angling the needle tip.
Gently rotate the infusion bag to mix—do not shake. Agitation may denature NAGLAZYME, rendering it biologically inactive. Therefore:
Label the infusion bag per your institution’s policy. Do not mix NAGLAZYME with other medicinal products.1
NAGLAZYME does not contain any preservatives; therefore, after dilution with saline in the infusion bag, any unused product or waste material should be discarded and disposed of in accordance with local requirements.1
IMPORTANT SAFETY INFORMATION
Life-threatening anaphylactic reactions and severe allergic reactions have been observed in some patients during NAGLAZYME infusions and up to 24 hours after infusion. If these reactions occur, immediate discontinuation of NAGLAZYME is recommended and appropriate medical treatment should be initiated, which may include resuscitation, epinephrine, administering additional antihistamines, antipyretics or corticosteroids. In patients who have experienced anaphylaxis or other severe allergic reactions during infusion with NAGLAZYME, caution should be exercised upon rechallenge; appropriately trained personnel and equipment for emergency resuscitation (including epinephrine) should be available during infusions.
As with other enzyme replacement therapies, immune-mediated reactions, including membranous glomerulonephritis have been observed. In clinical trials, nearly all patients developed antibodies as a result of treatment with NAGLAZYME; however, the analysis revealed no consistent predictive relationship between total antibody titer, neutralizing or IgE antibodies, and infusion-associated reactions, urinary glycosaminoglycan (GAG) levels, or endurance measures.
Caution should be exercised when administering NAGLAZYME to patients susceptible to fluid volume overload because congestive heart failure may result. Consider a decreased total infusion volume and infusion rate when administering NAGLAZYME to these patients.
Consideration to delay NAGLAZYME infusion should be given when treating patients who present with an acute febrile or respiratory illness. Sleep apnea is common in MPS VI patients and antihistamine pretreatment may increase the risk of apneic episodes. Evaluation of airway patency should be considered prior to the initiation of treatment. Patients using supplemental oxygen or continuous positive airway pressure (CPAP) during sleep should have these treatments readily available during infusion in the event of an infusion reaction, or extreme drowsiness/sleep induced by antihistamine use.
Pretreatment with antihistamines with or without antipyretics is recommended prior to the start of infusion to reduce the risk of infusion reactions. If infusion reactions occur, decreasing the infusion rate, temporarily stopping the infusion, or administering additional antihistamines and/or antipyretics is recommended.
During infusion, serious adverse reactions included laryngeal edema, apnea, pyrexia, urticaria, respiratory distress, angioedema, and anaphylactoid reaction; severe adverse reactions included urticaria, chest pain, rash, abdominal pain, dyspnea, apnea, laryngeal edema, and conjunctivitis. The most common adverse events (≥10%) observed in clinical trials in patients treated with NAGLAZYME were rash, pain, urticaria, pyrexia, pruritus, chills, headache, nausea, vomiting, abdominal pain and dyspnea. The most common adverse reactions requiring interventions are infusion-related reactions.
Spinal/cervical cord compression is a known and serious complication that is expected to occur during the natural course of MPS VI. Signs and symptoms of spinal/cervical cord compression include back pain, paralysis of limbs below the level of compression, and urinary or fecal incontinence. Patients should be evaluated for spinal/cervical cord compression prior to initiation of NAGLAZYME to establish a baseline and risk profile. Patients treated with NAGLAZYME should be regularly monitored for the development or progression of spinal/cervical cord compression and be given appropriate clinical care.
To report SUSPECTED ADVERSE REACTIONS, contact BioMarin Pharmaceutical Inc. at 1-888-906-6100, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information.
INDICATION
NAGLAZYME® (galsulfase) is indicated for patients with mucopolysaccharidosis VI (MPS VI; Maroteaux-Lamy syndrome). NAGLAZYME has been shown to improve walking and stair-climbing capacity.